Understanding how to find limiting reactant is fundamental in chemistry. The limiting reactant determines the maximum amount of product a chemical reaction can produce, and identifying it is critical for solving stoichiometry problems, performing laboratory experiments, and optimizing chemical reactions.
The limiting reactant, also known as the limiting reagent, is the reactant that is completely consumed first in a reaction, limiting the extent of the reaction and determining the theoretical yield of products. Mastering how to find the limiting reactant allows students and chemists to predict outcomes accurately, balance chemical equations, and understand the relationship between reactants and products.
This comprehensive guide explains how to find limiting reactant with moles, using grams, step-by-step procedures, determining excess reactants, and provides detailed examples for clarity. By the end of this article, you will be able to confidently calculate limiting reactants in any chemical scenario.
How to Find Limiting Reactant with Moles

Finding the limiting reactant using moles is one of the most straightforward methods in stoichiometry because chemical equations are written in terms of moles. This method relies on comparing the mole ratio of reactants used in the reaction to their coefficients in the balanced chemical equation.
• Write a balanced chemical equation – Begin by ensuring that the chemical equation is balanced, showing the correct mole ratios for each reactant. This is crucial because mole ratios dictate the relative consumption of reactants.
• Calculate moles of each reactant – Use the formula n=massmolarmassn = \frac{mass}{molar mass}n=molarmassmass if you have mass, or use given moles directly. Correct calculation ensures accuracy in identifying the limiting reactant.
• Determine the mole ratio for each reactant – Divide the number of moles available by the stoichiometric coefficient in the equation. This normalized ratio allows for a direct comparison between reactants.
• Identify the smallest ratio – The reactant with the smallest mole-to-coefficient ratio is the limiting reactant. This reactant is consumed first, controlling the reaction’s completion.
• Verify by calculating product formation – Use the limiting reactant to calculate the theoretical yield of products to confirm correctness. Cross-checking ensures accurate application.
• Analyze implications for excess reactants – Determine how much of the other reactants will remain unreacted. Understanding excess reactants helps in optimizing chemical usage and minimizing waste.
Using moles is efficient because it directly relates to the coefficients in the balanced equation and provides an easy method to determine the limiting reactant before calculating product yields.
Also Read:- How to Make Simple Syrup: The Ultimate Guide
How to Find Limiting Reactant with Grams

When reactants are given in grams, finding the limiting reactant requires converting grams to moles before comparing mole ratios. This method is widely used in lab experiments where reactants are measured by mass.
• Measure or note the mass of each reactant – Accurate mass measurement is essential for precise calculations. Use laboratory balances or given problem data.
• Convert grams to moles using molar mass – Apply the formula n=massmolarmassn = \frac{mass}{molar mass}n=molarmassmass for each reactant. This step ensures comparability in mole ratios.
• Write a balanced chemical equation – Identify coefficients for each reactant in the reaction. This step is critical for determining the correct mole ratios for comparison.
• Calculate the mole-to-coefficient ratio – Divide the moles of each reactant by its coefficient in the balanced equation. This determines which reactant is limiting.
• Identify the limiting reactant – The reactant with the smallest ratio is limiting and will determine the maximum product formed.
• Calculate remaining excess reactant – Subtract the amount consumed from the total available to find leftover mass. This ensures full understanding of reactant consumption.
Using grams allows chemists to work with real-world measurements while still accurately identifying the limiting reactant and predicting reaction outcomes.
Also Read:- How to Hold Chopsticks: The Complete Beginner’s Guide
How to Find Limiting Reactant Steps
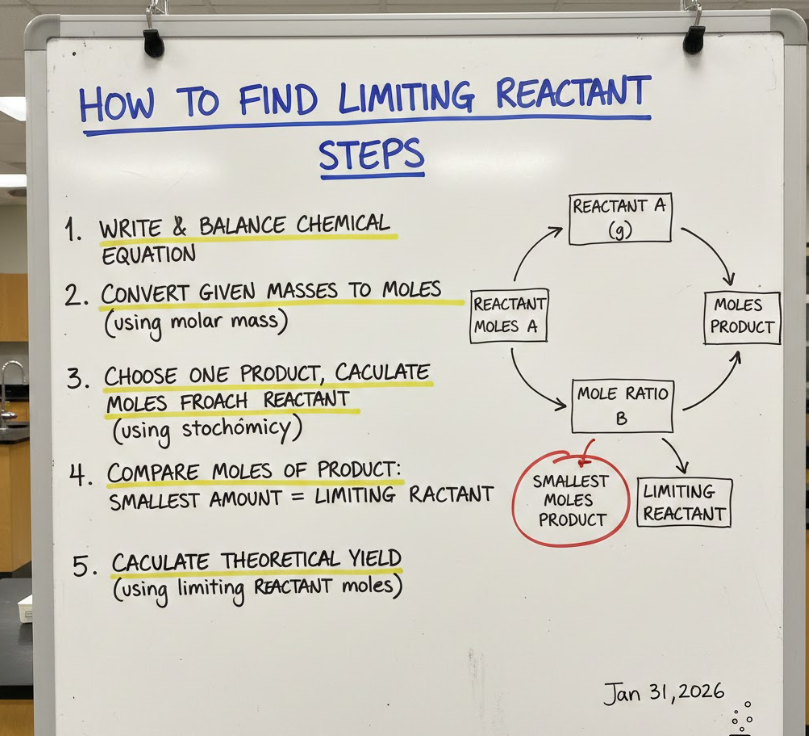
Understanding the step-by-step process to find the limiting reactant ensures a systematic approach and reduces errors. Following clear steps is essential in exams, labs, and stoichiometric calculations.
• Step 1: Write and balance the chemical equation – Ensure the reaction is balanced, as coefficients dictate the consumption of reactants. Accurate balancing prevents miscalculations.
• Step 2: Convert all given quantities to moles – Whether provided in grams, liters, or molecules, converting to moles standardizes units for comparison.
• Step 3: Divide moles by stoichiometric coefficients – Normalize each reactant by its coefficient to determine which is in the least proportion relative to the reaction.
• Step 4: Identify the limiting reactant – The reactant with the smallest normalized ratio limits the reaction. This is the critical factor in predicting product yield.
• Step 5: Calculate theoretical yield of products – Use the limiting reactant’s moles to determine the maximum amount of products possible. This validates calculations.
• Step 6: Determine excess reactant – Calculate the amount left after the reaction, which is important for resource efficiency and planning in experiments.
Following these steps allows chemists and students to systematically and accurately determine limiting reactants and reaction outcomes.
Also Read:- How to Fold a Fitted Sheet: The Complete Step-by-Step Expert Guide
How to Find Limiting Reactant and Excess Reactant
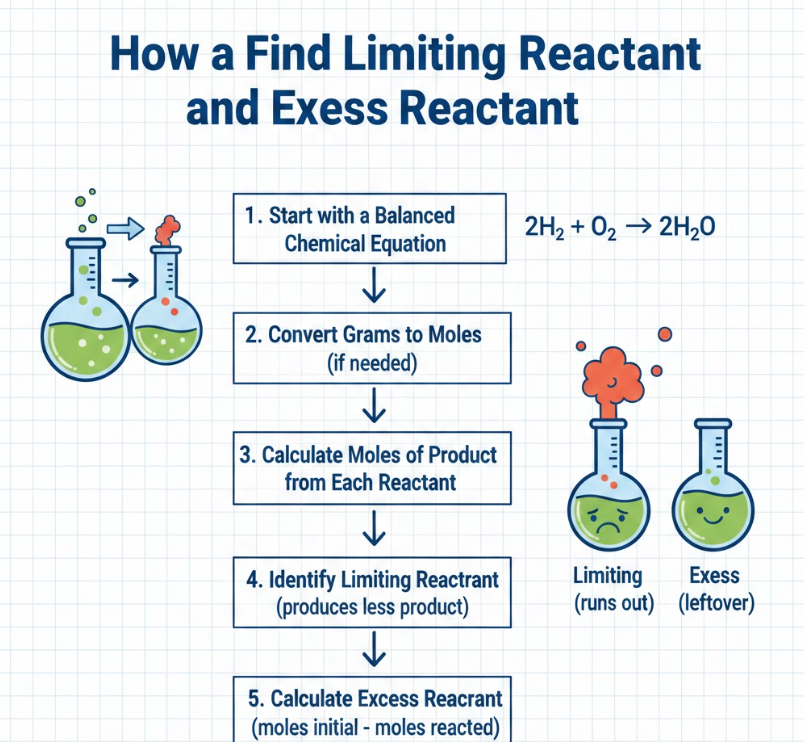
Understanding how to find limiting reactant and excess reactant is critical in lab experiments because it allows chemists to optimize chemical usage and minimize waste. While the limiting reactant determines product formation, the excess reactant shows what remains after the reaction.
• Identify the limiting reactant first – Use mole ratios or grams-to-moles conversions to determine which reactant is consumed first. This ensures accurate calculation of reaction extent.
• Calculate moles of product formed using the limiting reactant – The limiting reactant dictates the theoretical yield of the products. This step is crucial for predicting reaction outcomes.
• Determine moles of each reactant consumed – Multiply the limiting reactant’s moles by the stoichiometric ratio for other reactants. This helps track total consumption accurately.
• Calculate remaining excess reactant – Subtract the moles consumed from the initial moles available to find leftover quantities. This shows unused chemicals.
• Convert excess reactant back to grams if needed – Multiply remaining moles by molar mass to find the physical quantity remaining. Useful for lab measurements and reporting.
• Analyze implications for chemical efficiency – Understanding limiting and excess reactants allows optimization, cost reduction, and waste management in industrial or lab settings.
By identifying both limiting and excess reactants, chemists can design experiments efficiently, reduce waste, and predict outcomes accurately.
Also Read:- How to Eat Caviar: The Complete Expert Guide for Beginners and Enthusiasts
How to Find Limiting Reactant Example

Examples make the concept of limiting reactant clear and practical. Let’s examine how to find limiting reactant example with actual calculations.
• Example Reaction: 2H2+O2→2H2O2 H_2 + O_2 \rightarrow 2 H_2O2H2+O2→2H2O – Suppose 5 moles of H2H_2H2 and 2 moles of O2O_2O2 are available.
• Step 1: Calculate mole ratio – Divide available moles by coefficients: H2:52=2.5H_2: \frac{5}{2}=2.5H2:25=2.5, O2:21=2O_2: \frac{2}{1}=2O2:12=2.
• Step 2: Identify limiting reactant – O2O_2O2 has the smaller ratio, so it is the limiting reactant.
• Step 3: Calculate product formed – Using O2O_2O2 as limiting, moles of H2OH_2OH2O formed: 2×2=42 \times 2 = 42×2=4 moles.
• Step 4: Determine excess reactant – Moles of H2H_2H2 used: 2×2=42 \times 2 = 42×2=4, remaining H2=5−4=1H_2 = 5-4 = 1H2=5−4=1 mole.
• Step 5: Convert to mass if required – Multiply remaining moles of H2H_2H2 by molar mass (2 g/mol) to get 2 g leftover.
• Step 6: Summarize – Limiting reactant: O2O_2O2, excess reactant: H2H_2H2, theoretical yield: 4 moles H2OH_2OH2O.
This example illustrates practical steps, enabling students and chemists to solve similar problems with accuracy.
Also Read:- How to Make Fermented Spider Eye in Minecraft: Complete Guide
Conclusion
Mastering how to find limiting reactant is essential for chemistry success, whether in academics, lab work, or industrial applications. From calculations with moles and grams to step-by-step procedures, understanding both limiting and excess reactants allows accurate prediction of product yields, efficient use of chemicals, and improved experimental outcomes. Practical examples illustrate how these methods work in real reactions, ensuring clarity and confidence in solving stoichiometry problems.
FAQs: How to Find Limiting Reactant
Q: What is a limiting reactant?
A limiting reactant is the reactant completely consumed first, determining the maximum product formed.
Q: Can limiting reactants change if the reaction is incomplete?
Yes, incomplete reactions may leave initially limiting reactants unreacted, but in theory, it’s consumed first.
Q: Why is finding excess reactant important?
Knowing excess reactants helps minimize waste, optimize reactions, and predict leftover chemicals.
For More Info Visit Hola-Fly